The Pfizer vaccine should be routinely offered to women at any stage of pregnancy and vaccination should be recommended for women who are breastfeeding.
These are among the latest updates from the Gastroenterological Society of Australia (GESA) advice on the safety and efficacy of COVID-19 vaccines on people with underlying liver or gastrointestinal disease, including people with immunocompromised conditions or who are on immunosuppressants.
Other updates include the change in the recommended age group for the AstraZeneca from 50 plus to 60 plus, and the recommendation that where the Pfizer vaccine is unavailable, and where risk of COVID-19 infection outweighs the risk of the vaccine, AstraZeneca should be given to people of any age who have already had their first dose without any serious side effects.
The overall advice remains the same i.e. there are no concerns are anticipated on the safety and efficacy of COVID-19 vaccines on people with liver or gastrointestinal disease. This advice, updated 21 June 2021, offers an overview based on current information and recommendations from international liver and gastroenterology organisations including the AASLD, EASL, ILTS, BSG, ECCO and IOIBD.
The statement said large international registries are tracking the safety of these populations have thus far reported no increased risk of adverse events.
"Within the limitations of existing data, vaccinations do not appear to be associated with flares of activity of immune-related disease or transplant rejection. Irrespective of underlying immune-related disease or transplantation, patients should receive COVID-19 vaccination and disease activity should not impact the timing of vaccination or the choice of vaccine," it said.
It should be emphasised to patients that the Pfizer and AstraZeneca vaccines are not live-attenuated or replication-competent and therefore cannot cause COVID-19 or any other viral infection, the statement said.
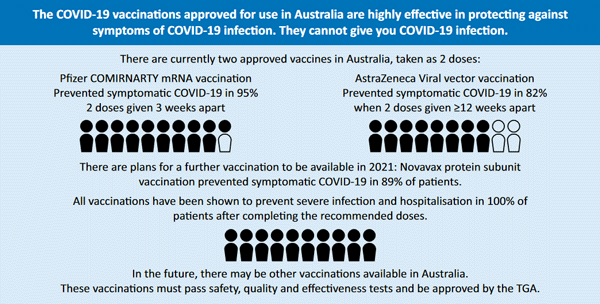
GESA has also published an information sheet on COVID-19 vaccine for community. The illustrated leaflet includes basic information on Pfizer and AstraZeneca vaccinations including dosage intervals, safety and efficacy. These COVID-19 vaccinations, approved for use in Australia, are recommended for people with, and taking medicines for, Inflammatory Bowel Disease (IBD), liver disease and liver transplants within the standard guidelines - Pfizer for those 16 years and above, AstraZeneca for people 60 years and over.
People who are pregnant, planning to be pregnant, or have allergies that cause breathing difficulties, or are under 16 years old, are advised to talk to their specialists before getting any COVID-19 vaccination.



