Direct-acting antiviral drugs (DAAs) to treat hepatitis C have proven to be a spectacular success, being able to cure more than 95% of people who have been living with the virus. However, a growing issue has implications for the design of future DAAs, and that is the ability of the hepatitis C virus–like all viruses–to mutate.
Mutant versions of viruses can “learn” to defeat the medications used to treat them by changing their structure or the way they interact with the bodies they infect. When they help the virus evade the effects of medication, we call these changes “drug resistance mutations” (DRMs), and they can make it much harder to cure infections. When a drug-resistant version of a virus becomes the dominant one, it can make a previously useful drug completely ineffective.
While multiple DRMs have been observed for all currently used antivirals, up until now it has not been known how this process actually allows the hepatitis C virus to overcome the effects of the medications. New research, published in Nature Communications in November, has started to dismantle this ignorance.
…the genes are cooperating or interfering with each other, and this teamwork can have a big impact on how things turn out, like in the case of drug resistance mutations in the hepatitis C virus.
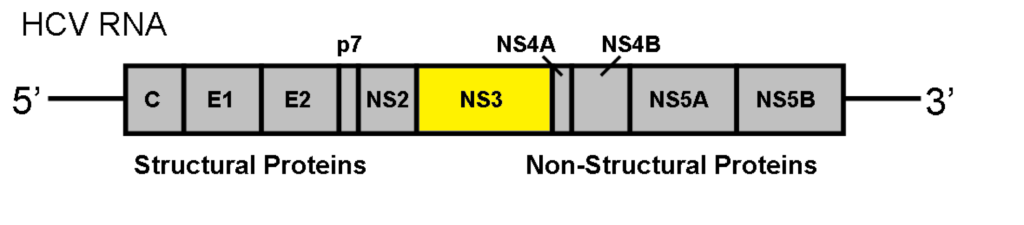
Many DAAs work by targeting a part of the hepatitis C virus called the nonstructural 3 (NS3) protein. The NS3 protein is created by hepatitis C to do a number of things, including allowing the virus to replicate in the human body. The research study, by Hang Zhang, Ahmed Abdul Quadeer (both based in Hong Kong) and Matthew R. McKay (based in Melbourne) looked specifically at the DAAs which target the NS3 protein, and found that a process call epistasis was significantly involved in growing drug resistance.
In simple terms, “epistasis” is like teamwork between different genes or mutations. Imagine you have a team of players in a game. The performance of one player might depend on how well another player is doing. If they work together well, the team performs better, but if there’s a problem between them, it might affect the whole team’s success.
In genetics, it’s a similar process. Epistasis is when certain genetic changes or mutations work together in a way that influences how they function or how they affect a trait. So, it’s like the genes are cooperating or interfering with each other, and this teamwork can have a big impact on how things turn out, like in the case of drug resistance mutations in the hepatitis C virus.
The study showed that hepatitis C is extremely good at mutating under the selective pressure of antiviral drugs, and that the easiest way for it to do this in a way that makes the drugs work less well is by modifying genes that, by epistasis, affect the NS3 protein.
This means that future antiviral research and development needs to focus on ways to overcome that. This might mean creating antivirals that affect other parts of hepatitis C than the NS3 protein, or perhaps ones which attack NS3 in a different way that mutations in other parts of the virus can’t overcome.
Last updated 10 April 2025
More from:
Enjoyed this article? Subscribe to be notified whenever we publish new stories.
Subscribe for Updates








