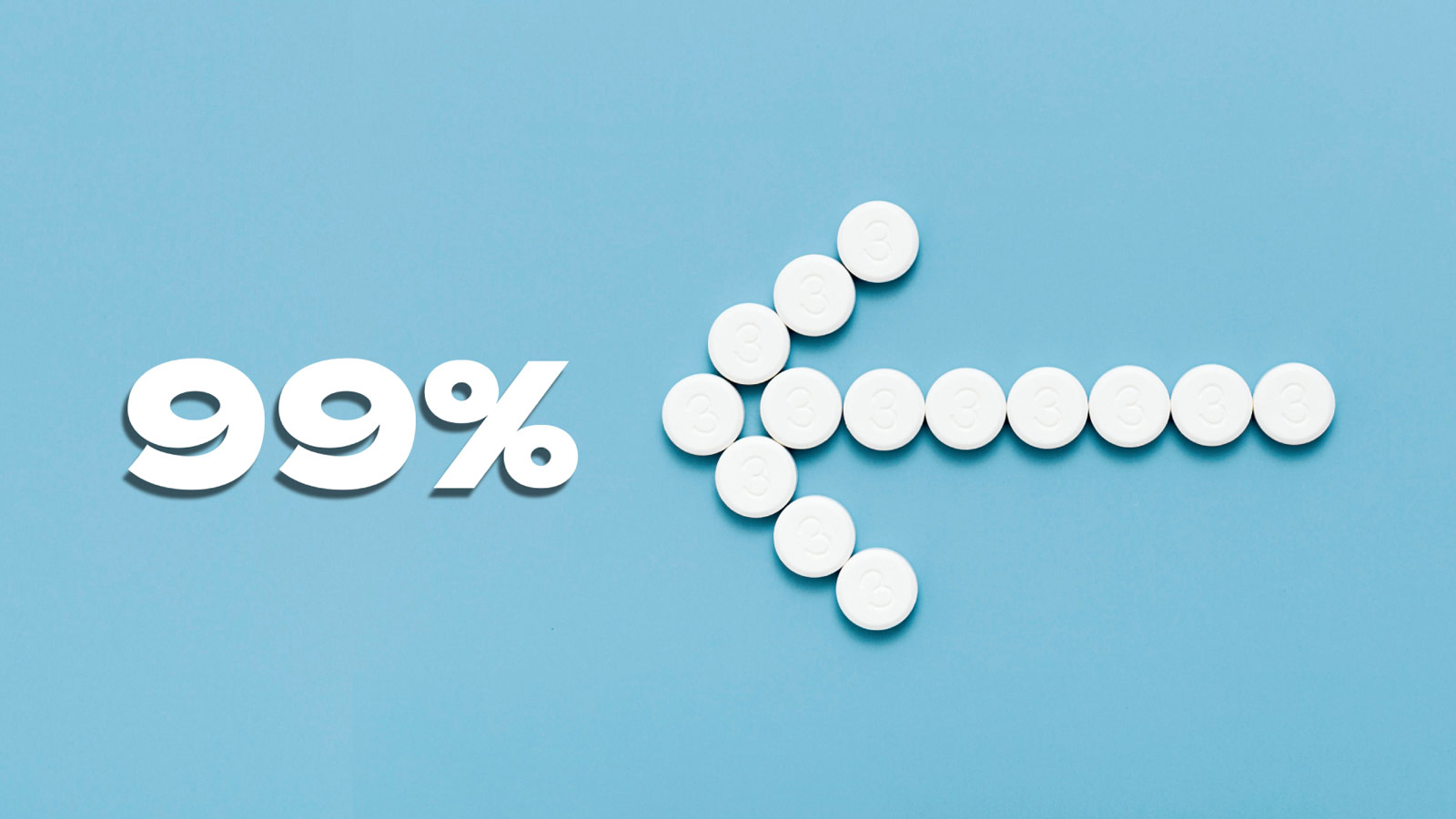
When we at Hepatitis SA talk with members of the community about hepatitis C treatment, we usually point out that the current available cure works for more than 95% of people, and has little no side-effects. This cure, consisting of a type of medication known as as direct-acting antivirals (DAAs), is available on the Pharmaceutical Benefits Scheme (PBS) in Australia.
A new, large-scale study by Gilead Sciences (one of the pharmaceutical companies that makes the medication, so they have a vested interest in its success) has demonstrated that the cure rate with sofosbuvir and velpatasvir (usually taken together over a 12-week period) is actually almost 99%.
The study, of more than 7000 patients in Australia, Canada, Europe, and the US, all treated with sofosbuvir/velpatasvir for hepatitis C virus infection reported a sustained virologic response (SVR) rate of 98.9%. SVR is when the virus is no longer detectable 12 to 24 weeks after treatment finishes.
Dr Anu Osinusi (Vice President, Virology. Head, Hepatitis, Respiratory and Emerging Viruses at Gilead Sciences) said the results were really exciting. “The study demonstrates that the high effectiveness of sofosbuvir and velpatasvir in real-world populations aligns with previous findings, reinforcing the global applicability of current HCV treatment guidelines.”

“We’ve seen that these pan-genotypic direct-acting antiviral agents for hepatitis C have consistently shown high SVR rates across diverse populations,” Osinusi said. “These results emphasize the broader applicability of these treatments worldwide.”

SVR rates were consistently high for both male and female patients, including those with genotype 3 (traditionally one of the harder genotypes of hepatitis C to cure) as well as those with cirrhosis.
“Our data show that even in the presence of more challenging factors like genotype 3 and cirrhosis, SVR rates remained above 97%, which is a strong indicator of the effectiveness of these therapies,” Dr Osinusi explained.
While SVR rates remained high across both sexes and age groups, the study also examined what is known as the time-to-treatment initiation (TTI), which is the interval between diagnosis and the beginning of therapy.
“The key message here is that minimizing the time to treatment initiation will result in better outcomes for all patients, reducing the risk of severe liver complications and increasing treatment rates across the board.
“The urgency to treat and minimise delays is crucial. Over time, it will result in fewer people having poor liver outcomes and will improve treatment rates for everyone infected with hepatitis C,” explained Dr Osinusi.
With cure rates at near-99% and better results from getting treated early, there has never been a better time to look at hepatitis C treatment.
Last updated 3 September 2025
More from:
Enjoyed this article? Subscribe to be notified whenever we publish new stories.
Subscribe for Updates