Reviewed by
Jo Morgan, Nurse Consultant, Hepatology Clinical Research, RAH
Dr. Damien Harding, Consultant Gastroenterologist & Hepatologist, LMH
Libby Cagney, Clinical Trials Manager Hepatology, FMC
MASLD (Metabolic Associated Steatotic Liver Disease) – also known as fatty liver – is a common condition which for some, is linked to an increased risk of heart disease and stroke, and for others can lead to complications of cirrhosis including liver cancer. Medical treatment for MASLD is developing rapidly with recent findings of two effective drugs, and further clinical trials are taking place.
South Australians were able to benefit from participation in clinical trials for treatment of MASLD, including the important ESSENCE study that demonstrated the benefit of one effective new treatment. (Sanyal et al., 2025) Involvement in a clinical trial may provide access to new treatments; it also provides participants with a higher level of clinical care, which many people with MASLD find beneficial.
While standard care for fatty liver in Australia remains focused on diet and exercise, participation in such clinical trials have enabled some Australians to access other emerging therapies.
For people with MASLD and other hard-to-treat conditions, understanding how clinical trials work can be invaluable
MASLD occurs when there is too much fat in the liver, usually due to conditions such as obesity, type 2 diabetes, high cholesterol or high blood pressure. The accumulation of fat in the liver can lead to inflammation and scarring, and over time can lead to cirrhosis, liver cancer and the need for a liver transplant.
For people with MASLD and other hard-to-treat conditions, understanding how clinical trials work can be invaluable. They provide a pathway to access potential new treatments while also contributing to advances in medical knowledge.

Clinical trials – what are they?
Clinical trials are research studies that test and evaluate new medical treatments to determine their safety and effectiveness. Participants may gain early access to promising therapies while helping researchers gather vital data.
Hepatology Clinical Research Nurse Consultant at the Royal Adelaide Hospital, Jo Morgan, explained: “Clinical trials are conducted under strict international guidelines and require ethical review and approval to ensure the safety of participants and the integrity of the data collected. Clinical trials can lead to the development of new treatments, improved patient care, and advancements in medical knowledge.
“The success of new Hepatitis C treatments highlights the power of clinical trials, with the world now moving toward the possibility of eradicating Hepatitis C.
“In Australia, people with fatty liver disease (like MASLD or MASH) can volunteer to try new drugs, lifestyle plans, or even exercise programs… all under close medical supervision.”
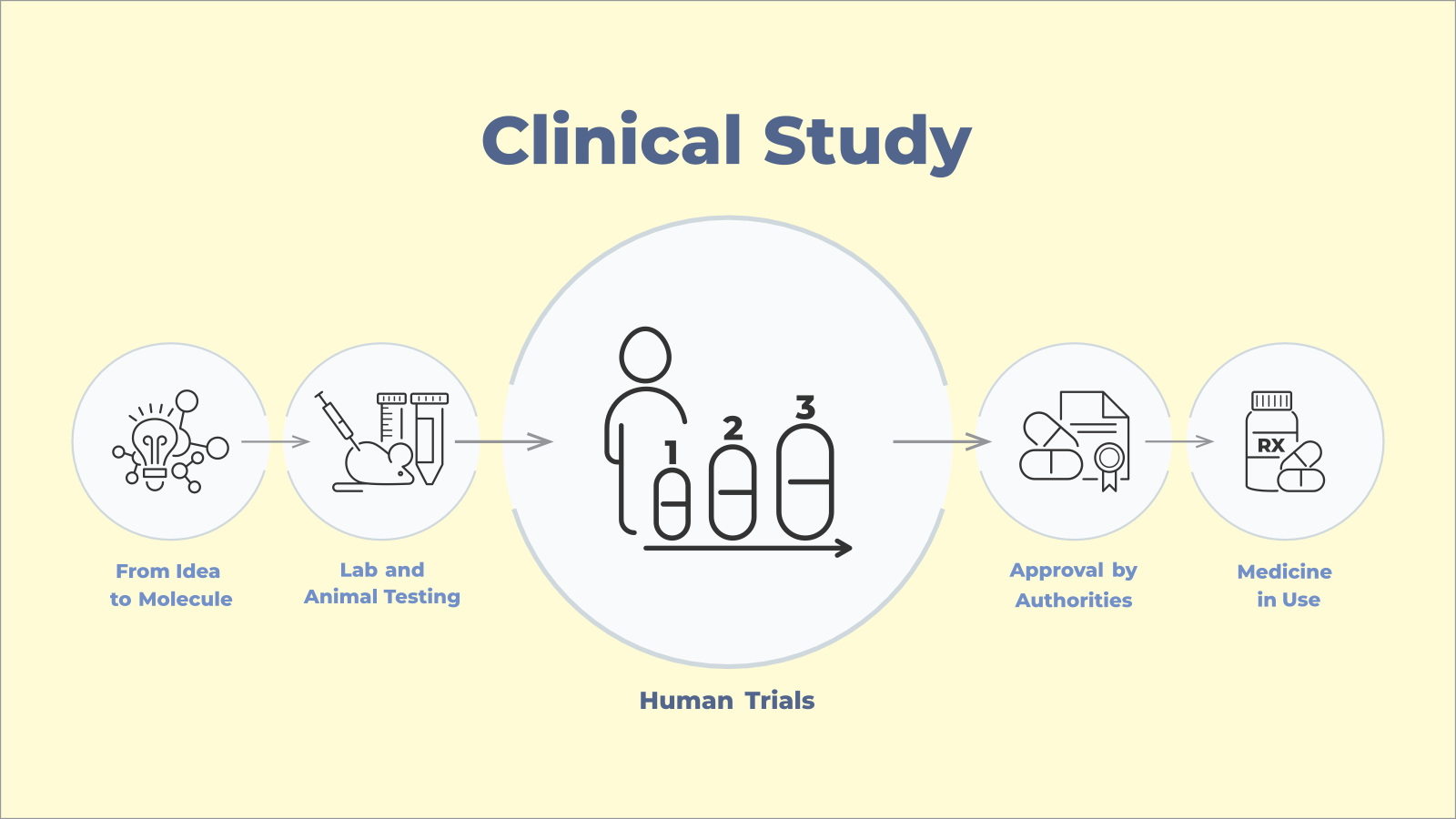
Clinical Trial Phases
Clinical trials are conducted in structured stages, usually referred to as “phases” each with a specific focus. It is useful to understand the focus of each phase and which phase you’re looking at, so you understand what you’re signing up for.
Clinical trials are commonly in four phases following pre-trial exploratory studies.
Phase 1 is when the medicine or treatment is first tested on humans. The goal of this phase is to establish safety of the drug or process being tested, and whether there are side-effects. This stage usually involves around 15-50 people. It is often very short, with minimal doses and may involve overnight stays to facilitate close monitoring.
Phase 2 focuses on testing effectiveness and tolerability. This stage usually involves 100 to 300 patients and treatment duration longer than Phase 1, but often still under a year. Participants usually have the medical condition that the treatment is meant to target. The trial may be a randomised controlled trial with more than one group of participants, with each group being given different treatments or dosages or it may be a non-randomised trial where all participants receive the treatment being tested at the same dosage.
Phase 3 trials are large-scale trials to confirm efficacy and compare against standard treatments. These are usually conducted in multiple locations with hundreds or thousands of people living with the disease to be treated. Participants may be 300 to 3000 and for drugs intended for long-term use, trial treatment period range from three months to three years. Phase 3 studies are often randomised, controlled and blinded. In other words, they would involve two or more test groups receiving different treatments and participants and/or researchers don’t know which group is receiving the new treatment. This allows the effectiveness, safety and dosage of the drug to be refined, and provides the information to doctors and consumers on how the drug is to be used safely.
Phase 4 clinical trials are conducted to track long-term effects of the treatment after it is approved by the Therapeutic Goods Administration (TGA) and made available for purchase. This Phase involves thousands of participants who are using the treatment – the aim being to assess how it works in the long-term, its interactions with other treatments, use in special population groups e.g. during pregnancy, and potential alternative uses.
For more details: https://clinicaltrialshub.htq.org.au/blog/understanding-the-clinical-trial-phases/


Opportunities for People with Fatty Liver Disease
According to Jo Morgan, new medications for MASLD are being actively tested. “These clinical trials are investigating whether new drugs can reduce liver fat, stop inflammation, and prevent or reduce scarring. Recruitment aims to include a wide range of participants. Don’t be discouraged by barriers such as distance or language—interpreters can be arranged, and travel reimbursements may be available for regional patients.”
MASLD is now one of the most common liver conditions worldwide. For some people, particularly those with advanced scarring, it can lead to serious complications. In Australia, fatty liver disease is now recognised as a leading cause of liver transplantation. (Howell et al., 2022; Mahady & Adams, 2018).
This makes early detection and the development of new treatments increasingly important. Most people, however, can manage their liver health with the right care.
Where to Find Clinical Trial Opportunities
Royal Adelaide Hospital
Hepatology (Liver) Clinical Trials Nurses
📧
📞 0403 108 032 or (08) 7074 2190
Flinders Medical Centre
Libby Cagney – Clinical Trials Manager, Hepatology
📧
📞 (08) 8204 7544 | Fax: (08) 8204 6420
Lyell McEwin Hospital
Brenda Trezona – Clinical Trials Clinical Trials Coordinator
📧
📞 (08) 8282 0666
Paul Knudsen – Clinical Trials Manager
📧
📞 (08) 8282 0219
Online registries:
- www.anzctr.org.au (Australia & New Zealand Clinical Trials Registry)
You can also talk to your liver specialist or GP to help you find a suitable trial.
References
Australian Clinical Trials. (n.d.). About clinical trials. National Health and Medical Research Council. Retrieved September 30, 2025, from https://www.australianclinicaltrials.gov.au
Chalasani, N., Younossi, Z., Lavine, J. E., Charlton, M., Cusi, K., Rinella, M., … & Sanyal, A. J. (2023). The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology, 77(5), 1797–1835. https://pubmed.ncbi.nlm.nih.gov/28714183/
Howell, J., Balderson, G., Hellard, M., Strasser, S. I., Fink, M., & Doyle, J. S. (2022). Hidden burden: The changing face of liver transplantation in Australia and New Zealand. Transplantation Direct, 8(12), e1394. https://pmc.ncbi.nlm.nih.gov/articles/PMC10109460/ | https://europepmc.org/article/MED/37077731
Mahady, S. E., & Adams, L. A. (2018). Burden of non-alcoholic fatty liver disease in Australia. Journal of Gastroenterology and Hepatology, 33(1), 171–181. https://pubmed.ncbi.nlm.nih.gov/29851153/
Sanyal, A.J., Newsome P.N., Kliers I., Østergaard L.H., Long M.T., Kjær M.S., …& Ratziu V. (2025). Phase 3 Trial of Semaglutide in Metabolic Dysfunction-Associated Steatohepatitis. New England Journal of Medicine, 392(21), 2089-2099. https://doi.org/10.1056/NEJMoa2413258
Younossi, Z. M., Koenig, A. B., Abdelatif, D., Fazel, Y., Henry, L., & Wymer, M. (2016). Global epidemiology of nonalcoholic fatty liver disease—Meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology, 64(1), 73–84. https://doi.org/10.1002/hep.28431
Burnet Institute. (2022). The increasing burden of potentially preventable liver disease among adult liver transplant recipients in Australia and New Zealand. Burnet Institute. Retrieved September 30, 2025, from https://www.burnet.edu.au
Last updated 9 December 2025
More from: