People living with chronic hepatitis B (CHB) whose treatment had been deferred may be offered therapy as professional bodies review their guidance following the release of new guidelines by the World Health Organization (WHO) in 2024. Momentum is growing to simplify and expand treatment criteria so that more people with chronic hepatitis B will be treated to prevent adverse outcomes.
While issues around side-effects and resistance still exist, effective new drugs have been developed in the last 20 years that are more well-tolerated and have higher barriers to resistance. This, together with high prevalence and rising deaths, has prompted a review of guidelines so that more people with CHB, especially those in vulnerable communities with less access to medical services, may be treated in order to save lives and reduce transmission.
BRIEFLY
The new WHO guidelines on hepatitis B, launched on 30 March 2024, have the following priority areas:
- expanded treatment eligibility, and inclusion of adolescents;
- alternative antiviral therapy regimens;
- expanded eligibility for antiviral prophylaxis among pregnant women to prevent mother-to-child transmission;
- HBV diagnostics – use of point-of-care (POC) DNA assays and reflex HBV DNA testing;
- testing for hepatitis delta (hepatitis D) coinfection.
The guidelines were developed according to procedures established by the WHO Guidelines Review Committee. Clinical recommendations were formulated by a regionally representative and multidisciplinary Guidelines Development Group at a meeting held in May 2023.
Read the World Hepatitis Alliance community briefing paper.
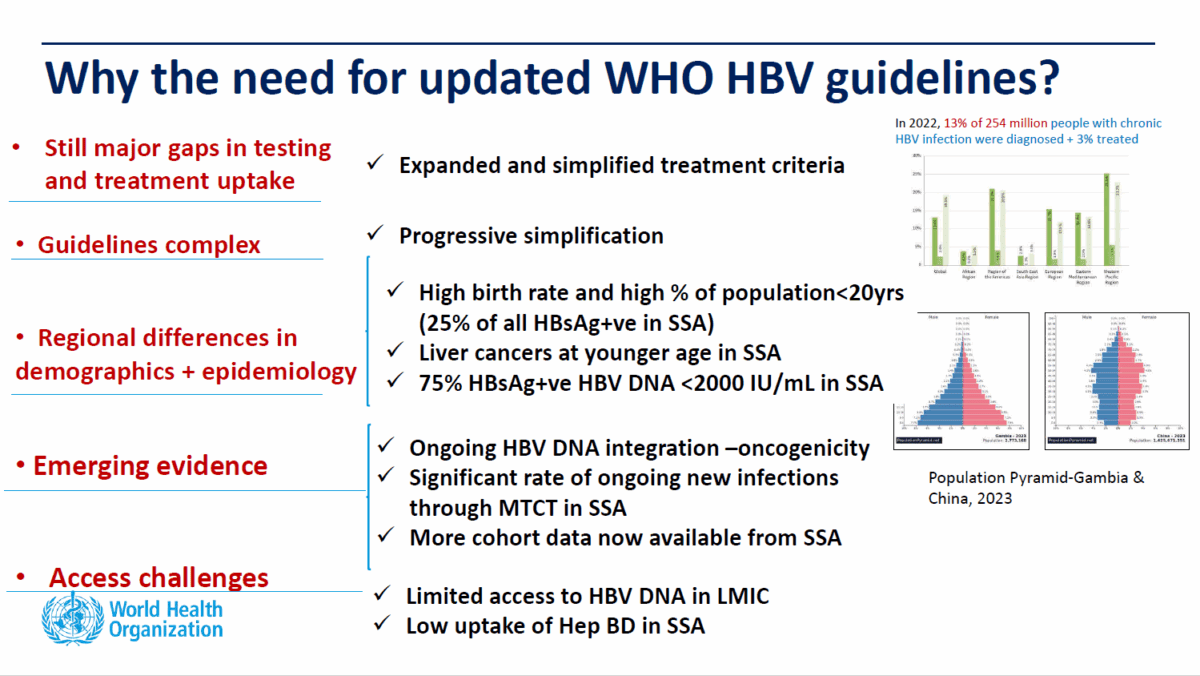
Globally, hepatitis B accounts for the vast majority of hepatitis deaths and new infections. Over 300 million people live with chronic hepatitis B or C, with hepatitis B accounting for over 254 million. Each year, over 1.3 million die from hepatitis-related liver disease, including cancer. Of these, 1.1 million are due to hepatitis B. Of the 2.2 million new hepatitis infections each year, 1.23 million are hepatitis B.
With only 13 out of a 100 people with CHB diagnosed, 221 million remain unidentified, with no monitoring or care. Of the 33 million who are diagnosed, only 3 out of 100 are receiving treatment. The numbers in Australia are better, but there remain 75,000 undiagnosed out of the estimated 220,000 Australians living with chronic hepatitis B and treatment rates not meeting targets.
To add to the complexity, of the 254 million around the world living with hepatitis B, 12 million are also infected with hepatitis D, a virus that exists only in the presence of the hepatitis B virus and accelerates progression of liver disease with devastating outcomes. A recent survey by the World Hepatitis Alliance has found very low levels of awareness about hepatitis D and limited access to testing and treatment.
Hepatitis Delta
One stand-out recommendation in the 2024 WHO guidelines is reflex testing for hepatitis D in everyone living with chronic hepatitis B. This is the first time WHO guidelines have discussed hepatitis D. Changes to testing guidelines include:
- The use of non-invasive tests to determine eligibility for treatment and point-of-care tests for hepatitis B status are recommended in situations where access to other traditional assessment tools are limited.
- Testing for all pregnant women
- Reflex testing for hepatitis D
Fibrosis and Co-infections
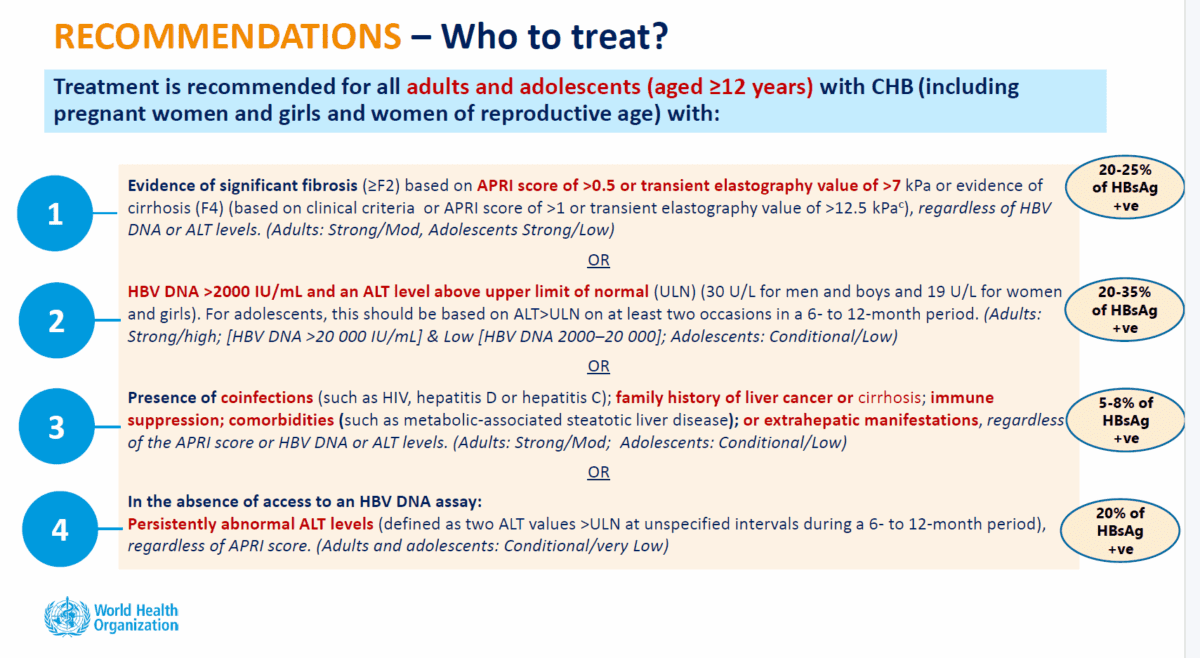
Other updated recommendations include treatment for all adults and adolescents with chronic hepatitis B, including pregnant women and girls and women of reproductive age, who meet one of the following criteria:
- stage 2 fibrosis or above
- high viral load (over 2000 IU/mL) and above normal ALT
- regardless of test scores, presence of co-infections (HIV, hepatitis D or hepatitis C), family history of liver cancer or cirrhosis, immune suppression, other conditions such as metabolic dysfunction-associated fatty liver disease (MAFLD), or symptoms outside of the liver,
- where HBV DNA testing is not available, persistently abnormal ALT levels
- treatment for any HBV-positive pregnant women in settings where hepatitis B DNA and e Antigen testing is not available.
The significant change – while maintaining current criteria for treatment – is the recommendation to expand treatment eligibility to include people with stage 2 fibrosis for whom treatment is deferred under previous guidelines.
Simplified Testing
The new WHO guidelines provides for the diagnosis of fibrosis using non-invasive point of care APRI tests or a fibrosis scan, thus simplifying the process. With the expansion of treatment criteria to stage 2 fibrosis, the APRI test becomes a more reliable tool as it is significantly more accurate in picking up positives in the earlier stages of liver scarring (fibrosis) than in more advanced stages (cirrhosis).
By allowing alternatives to more costly or complex testing as assessment tools, access to treatment is simplified, a feature especially useful for low- and middle-income countries with limited access to HBV DNA testing. This allows for easier entry to treatment to prevent the onset of cirrhosis and liver cancer.
People with co-infections such as HIV, hepatitis D, diabetes or MAFLD, or a family history of liver cancer, are all recommended for treatment as they have a higher risk the liver disease progressing. In addition, recommendation for treatment is for the first time, open to adolescents i.e. any person over the age of 12.
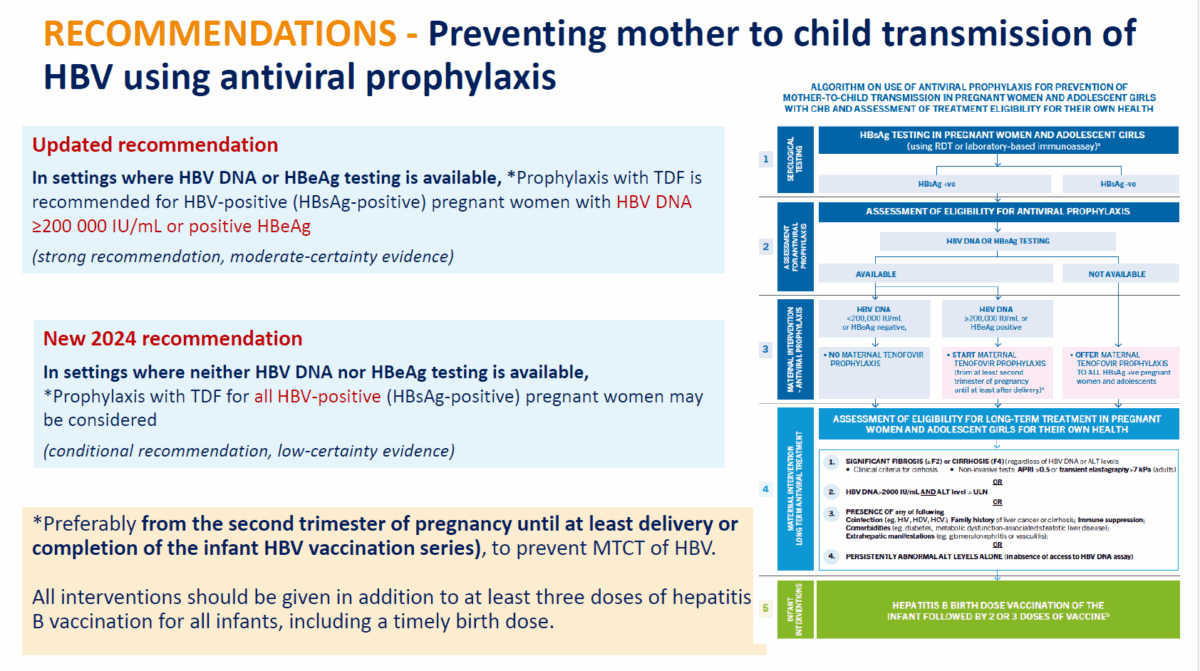
Pregnancy
The new approach to hepatitis B management in pregnant women recommends hepatitis B antigen or DNA testing for all pregnant women and antiviral therapy for women who meet the traditional criteria – HBV DNA ≥200,000 IU/mL or a positive hepatitis B e-Antigen (HBeAg). In addition to that however, a new recommendation gives all HBsAg-positive pregnant women in settings with no access to the above tests, the option to take anti-viral drugs to prevent mother to child transmission, should they choose to.
This addresses the issue of barriers to accessing hepatitis B DNA testing and hepatitis B eAntigen testing that may prevent otherwise eligible women from getting preventive treatment. Treatment is recommended from the second trimester of pregnancy until at least delivery or completion of the infant HBV vaccination.
The Hepatitis B Foundation, issued a position statement calling for treatment to be made available to people living with chronic hepatitis B
Treatments
In terms of treatment regimens, the guidelines maintain the recommendation of using entecavir and tenofovir disoproxil fumarate (TDF) as preferred regimens with the alternative of using TDF in combination with other drugs where its availability is limited. New recommendations include:
- reserving the more costly new drug, tenofovir alafenamide fumarate (TAF), for people with bone or kidney disease,
- using only TDF and TAF for MTCT preventive treatment as entecavir has been associated with foetal defects, and
- using entecavir for children between three and twelve and TAF for those aged 12 or over.
Community Response
Following the release of the new WHO guidelines, the Hepatitis B Foundation, issued a position statement calling for treatment to be made available to people living with chronic hepatitis B. The statement was crafted by 28 prominent researchers and health professionals with an additional 120 signatories.
The World Hepatitis Alliance and the Hepatitis B Foundation have both produced community-friendly, easy to read information documents explaining the key changes. The WHA community briefing paper also includes page references to the official WHO document to assist in reading and understanding the full guidelines. Click on the images or links below to read the documents.

The World Hepatitis Alliance community briefing paper gives a summary of the new WHO guidelines on the prevention, diagnosis, care and treatment for people with chronic hepatitis B infection. References to the WHO document are provided with the summary of each section. Click here or on the image to read.

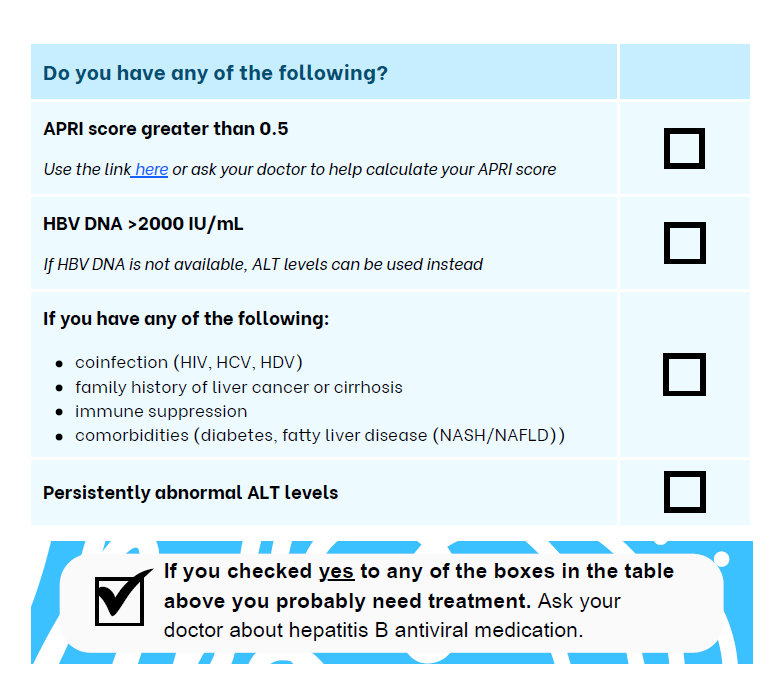
The Hepatitis Foundation fact-sheet on the WHO guidelines include a simple checklist for people living with chronic hepatitis B to assess if they should talk to their GPs about treatment. Click on the image or here to read.
Note: There are as yet no updates from Australian institutions, so practitioners may or may not be open to be approached for discussion.
Sources
- Bridging the Gap to a Functional Cure for Hepatitis B, Dr Su Wang, 21 April 2025 – https://clinicaloptions.com/activities/infectious-disease/functional-cure-for-hepatitis-b/89165/viewactivity
- World Health Organisation Guidelines for the prevention, diagnosis, care and treatment for people with chronic hepatitis B infection – https://www.who.int/publications/i/item/9789240090903
- WHO hepatitis B guidelines on the prevention, diagnosis care and treatment for people with chronic hepatitis B infection – Technical Briefing Webinar – https://www.youtube.com/watch?v=cRPhH0E_Euc
Credits
- Slides courtesy of the WHO Global Webinar: Technical Briefing on 2024 WHO Hepatitis B Guidelines on Diagnosis Treatment and Service. https://www.youtube.com/watch?v=cRPhH0E_Euc
- Header image composite of artwork from the following: Family by Tetiana Garkusher via iStock; Liver by anna42f via iStock; Virus – Елена Верхотурова via iStock
Last updated 18 August 2025
More from:
Enjoyed this article? Subscribe to be notified whenever we publish new stories.
Subscribe for Updates