Suppose for a moment that you are one of thousands of people around the world each year who confront the possibility that they may have contracted the hepatitis C virus. You’re worried, perhaps confused, and have a million other things on your mind. Where do you start?
You’ll need a blood test, so you go to a GP, only to find you need to make another appointment—usually at another location—to have the blood test done. And then, after an agonising wait (an average of a week), you go to another appointment with the GP to find out the result.
If you discover you have hepatitis C antibodies, you’ll need to go through this process again—blood test, then return to the GP for the result—to find out if you have a hepatitis C infection. This is because antibodies tell us if you’ve had the virus in your body, but don’t tell us if you have a current infection.
Although interferon-based treatments have thankfully been displaced by effective, short-term and very tolerable oral therapies, there are still significant obstacles to hepatitis C treatment in Australia. Described above is the process to determine a person’s hepatitis C status under Medicare rules, and it is no wonder that many people don’t make it to the final appointment to find out their diagnosis: life just gets in the way! If you’re a person who finds blood tests difficult and the thought of needles makes you go dry in the mouth, it’s possible you might not even make it to the first blood test for a very long time if at all.
Imagine if all of these encounters could be compressed into a single appointment: a person could have a test with blood taken from a finger prick rather than a needle, get the result, and be prescribed treatment for hepatitis C all in one visit to their healthcare provider. The PROMPt study aimed to make this a reality in three locations across Adelaide during a 12-month trial period. The launch of the study was covered in our magazine in mid-2021 (see issue 90).
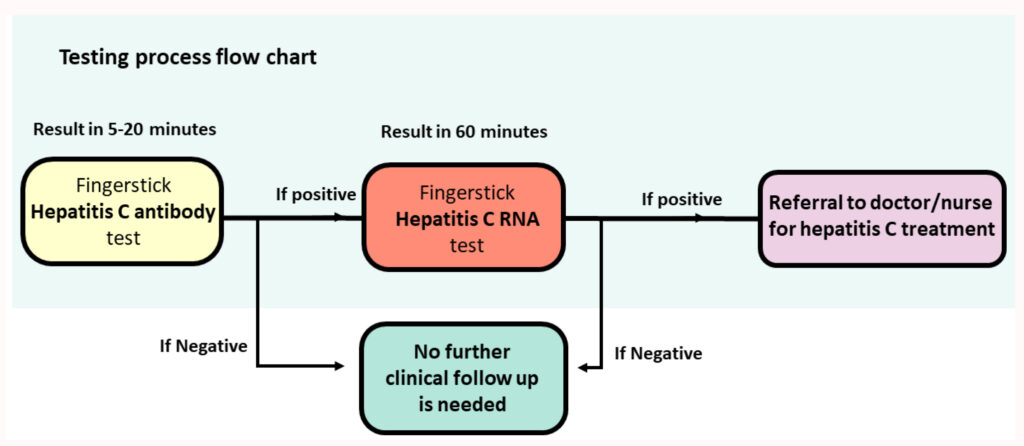
PROMPt was run at the Adelaide Remand Centre, at inpatient Alcohol & Other Drug Services, and at a mental health inpatient unit. Participants were offered an HCV diagnostic test using a quick fingerstick antibody (Ab) assay. Participants who tested HCV-Ab-positive were then offered a fingerstick HCV RNA assay run on the GenXpert machine.
Project staff, including peer educators, provided HCV education, pre- and post-test counselling, and linkage to care at the time of diagnosis.
Participants who tested HCV RNA positive underwent post-test counselling, harm reduction education and were linked to treatment. Linkage to care consisted of site staff being notified of the positive HCV RNA results, and participant details being sent to the local viral hepatitis nurse for management and treatment.
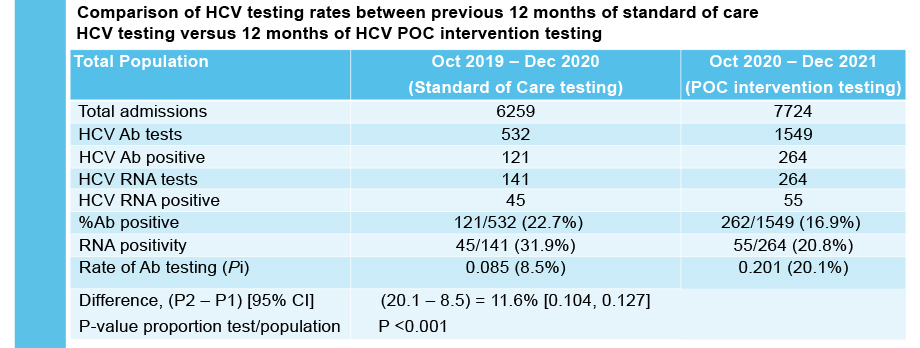
The study results demonstrate that it is feasible to provide HCV POC testing in the priority settings of mental health, prisons and AOD services. HCV POC testing increased overall rates of HCV testing as compared to the previous 12 months of standard of care testing.
Combined HCV antibody and RNA testing at the point of care has resulted in a significant testing scale-up at each service. The researchers found high acceptability of POC testing at each service.
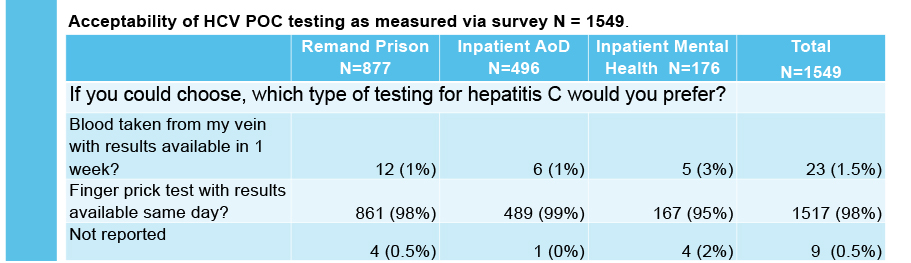
Working with site staff and local viral hepatitis nurses was extremely effective in facilitating HCV treatment. PROMPt shows that this approach is an excellent way to improve testing rates and ensure people get the treatment and support they need if they are living with hepatitis C .