An Australian expert panel of medical specialists have recommended that all people receiving immunosuppression cancer therapy be screened for hepatitis B.
If you have ever been infected by hepatitis B, cancer treatment which suppresses your immune system can allow the virus to reactivate – even if your body had dealt with it successfully before. Reactivation can lead to liver failure, death or sub-optimal cancer treatment.
This reactivation phenomenon is now well understood among health care providers but until recently, there has been no agreed guidelines for practitioners on the management of hepatitis B under such circumstances.
The risk of reactivation depends on the length and extent of the cancer treatment, the drugs and treatment regimen used, underlying hepatitis B viral activity and the extent of liver disease.
Antiviral therapy is effective and known to prevent reactivation but guidance is needed for practitioners on the most appropriate way to use it. Over or under usage could lead to unwelcome side effects or hepatitis B reactivation, respectively.
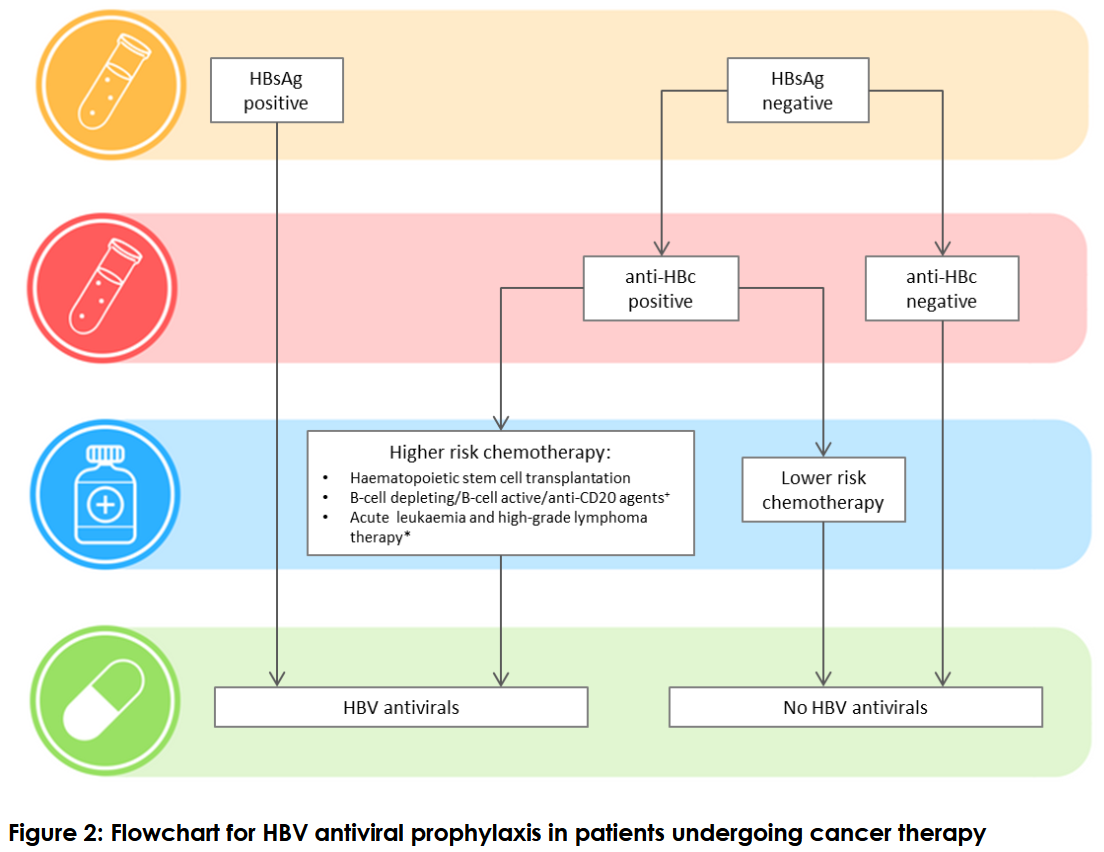
The April 2019 consensus statement – Hepatitis B management during immunosuppression for haematological and solid-organ malignancies – made 27 recommendations including:
- all patients undergoing therapy for blood cancers and solid tumours be tested for hepatitis B infection,
- the treating haematologist or medical oncologist prescribing cancer therapy be responsible for hepatitis B testing,
- hepatitis B screening include testing for hepatitis B surface antibody, surface antigen and core antibody,
- all patients found to have chronic hepatitis B be put on antiviral prophylaxis
- patients without chronic hepatitis B but have had past infection, be assessed for reactivation risk based on the cancer therapy regimen,
- antiviral prophylaxis should start as soon as possible but should not delay cancer therapy,
- people without previous exposure to hepatitis B do not need antiviral prophylaxis but if they lack hepatitis B immunity, they be offered vaccination six months after completion of cancer therapy and the underlying disease is controlled
The consensus statement recommends antiviral prophylaxis begins as soon as possible without delaying cancer therapy and that all those with chronic hepatitis B be referred to a viral hepatitis specialist for routine assessment.
Guidance is further given on when to stop antiviral treatment and how patients should be monitored.
The statement also recommends that testing, antiviral prophylaxis commencement and cessation for children follow the same approach as for adults.
The consensus statement was prepared by an expert panel of medical specialists in infectious diseases,hepatology, haematology, oncology and paediatrics, and representatives from the Australasian Society for Infectious Diseases, the Gastroenterological Society of Australia (Australian Liver Association), the Haematology Society of Australia and New Zealand, the Medical Oncology Group of Australia, and the Australasian Society for HIV, Viral Hepatitis and Sexual Health Medicine.
The statement is endorsed by the Australasian Society for Infectious Diseases, Gastroenterological Society of Australia, Haematology Society of Australia and New Zealand, Medical Oncology Group of Australia, and Australasian Society for HIV, Viral Hepatitis and Sexual Health Medicine.
Last updated 24 May 2024
More from:
Enjoyed this article? Subscribe to be notified whenever we publish new stories.
Subscribe for Updates